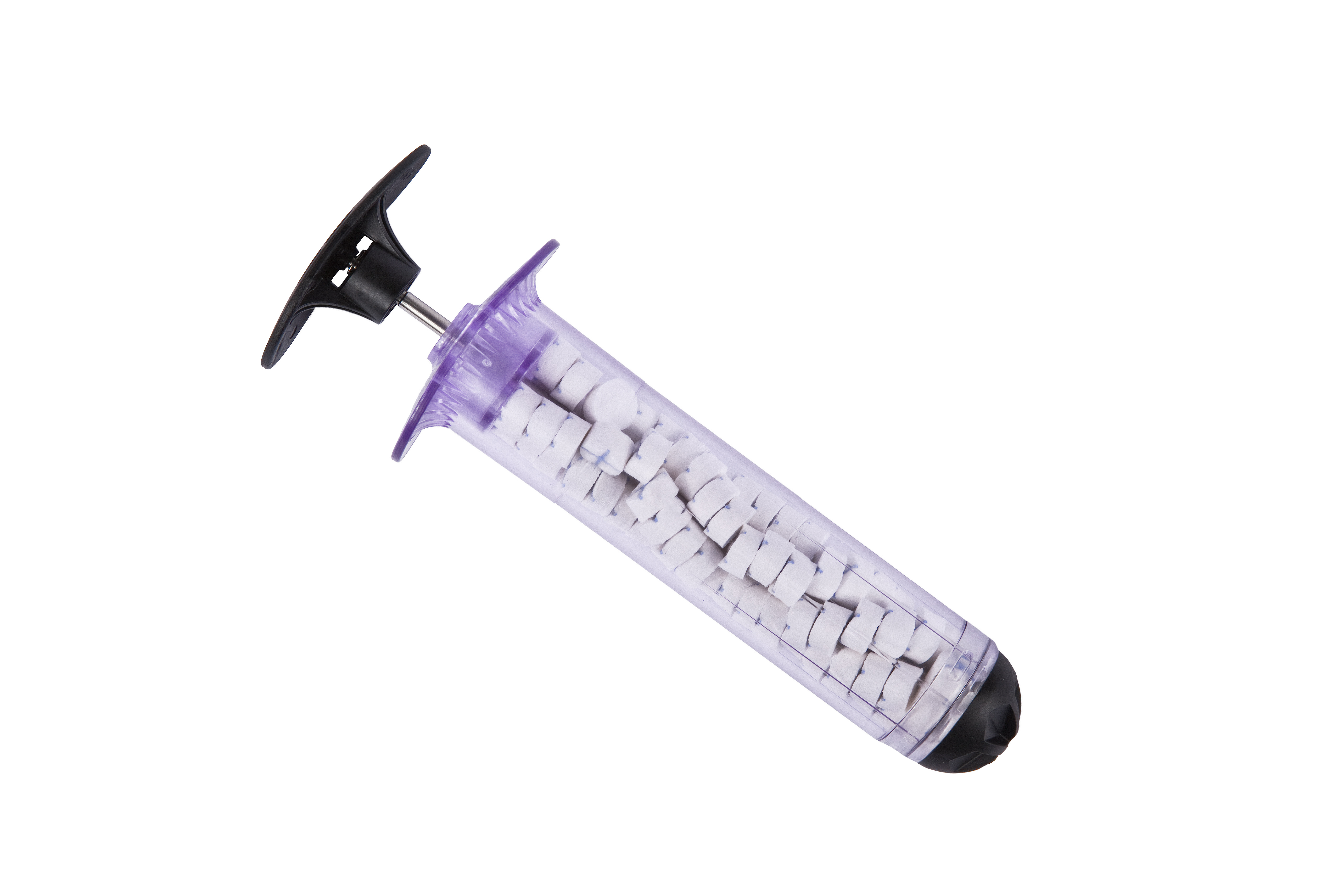
The XSTAT hemorrhage control device is now featured in the November 1 2020 edition of the Journal of Special Operations Medicine Online Newsletter. This device profile discusses the origins, advantages and uses of this innovative mini sponge technology. Access the JSOM Newsletter here or download the .pdf below.
Author: kate_revmedx
TX® Series added to CoTCCC list of recommended limb tourniquets
RevMedx, Inc., a leader in groundbreaking solutions for the control of severe bleeding, today announced that the CoTCCC has added the TX Series ratcheting tourniquets to its list of recommended Limb Tourniquets. The TX series joins the XSTAT® injectable hemostatic on the TCCC list of recommended hemorrhage control devices for casualty care.
Andrew Barofsky, President and CEO of RevMedx, Inc., stated, “We are pleased that yet another RevMedx product is now part of the TCCC guidelines. Our company is dedicated to adding innovative solutions for first responders and medics around the world.”
The United States Army Institute of Surgical Research reports that 30 to 40 percent of civilian deaths by traumatic injury are the result of hemorrhage, many of which occur before the patient reaches an emergency care facility. For victims of violence or accidents, early control of severe bleeding may prevent shock and save a life.
While there are a variety of tourniquets on the market, their narrow width and windlass tightening mechanism pose limitations to ease of use and patient comfort.
Some research suggests that wider tourniquets have been known to provide better patient comfort during application and transport as well as having the potential to minimize tissue and nerve damage during application. The TX2 and TX3 (2 and 3 inches wide respectively) are the widest non-pneumatic tourniquets recommended by TCCC. With an intuitive and simple ski boot ratchet design, the TX series enable fast application with minimal training.
About RevMedx
Based in Wilsonville, Oregon, RevMedx is a privately held medical device company whose goal is to design, develop, and manufacture innovative medical products that save lives. Our product line includes XSTAT, XGAUZE, AIRWRAP, PARABELT, SHARKBITE Blast Injury Kits, and TX Series Ratcheting Tourniquets. Additional information about RevMedx and our products can be found at www.revmedx.com.
RevMedx receives CE Mark for XGAUZE® self-expanding gauze.
Manufacturer of the revolutionary XSTAT® technology will now have a second sterile wound dressing available in Europe and other International markets.
RevMedx, Inc., a global leader in innovative devices that control severe bleeding, today announced it has received CE (Conformité Européenne) Mark Certification for the XGAUZE wound dressing in Europe. XGAUZE, a Class I sterile multi-layer wound dressing, contains an embedded layer of compressed cellulose sponges that expand upon contact with blood or fluid.
XGAUZE is standard issue in selected US Armed Forces medical kits and has several hundred documented treatments since its release in 2015. Using expanding sponge technology similar to RevMedx’s XSTAT injectable device, compressed sponges embedded in XGAUZE rapidly absorb blood and expand to ten times their original size. One expanded XGAUZE fills about the same volume as three standard gauze dressings. XGAUZE may provide significant advantages for wound-packing and treatment of certain types of traumatic injuries seen in pre-hospital settings.
Andrew Barofsky, President and CEO of RevMedx, Inc., stated, “CE Mark on our XGAUZE product now makes our entire portfolio available to emergency responders and surgeons in the European Union and around the world. This certification clearly indicates that XGAUZE and our supporting quality system have met the essential requirements for product safety, performance and usability, in compliance with rigorous requirements set by the EU’s Medical Device Directive (MDD).”
For victims of violence or accidents, early control of severe bleeding may prevent shock and may be life-saving. The United States Army Institute of Surgical Research reports that 30 to 40 percent of civilian deaths by traumatic injury are the result of hemorrhaging; of those deaths, 33 to 56 percent occur before the patient reaches an emergency care facility.
About RevMedx
Based in Wilsonville, Oregon, RevMedx is a privately held medical device company whose goal is to design, develop, and manufacture innovative medical products that save lives. Our product line includes XSTAT, XGAUZE, AIRWRAP, PARABELT, SHARKBITE Blast Injury Kits, and TX Series Ratcheting Tourniquets. Additional information about RevMedx and our products can be found at www.revmedx.com.
XSTAT featured in Business Insiders “Top 13 Medical Advances”
XSTAT featured in Business Insiders “Top 13 Medical Advances”.
VP of Strategic Development, John Steinbaugh interviewed by Tech Briefs
VP of Strategic Development, John Steinbaugh interviewed in Tech Briefs’ podcast series, “Here’s an Idea™” on XSTAT® and how expanding sponges are changing the world.